Description
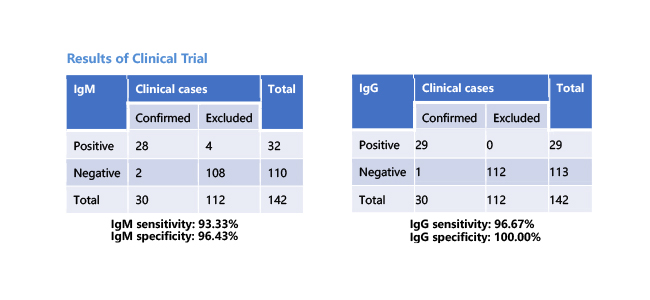
- More advanced technology: Immunofluorescence assay, better sensitivity, and specificity than colloidal gold assay.
- Multiple sample types: whole blood, serum, and plasma.
- Two joint inspections: Detection of specific antibodies IgM and IgG separated at the same time.
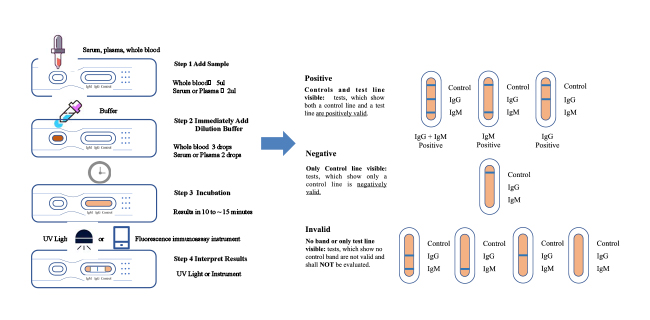
- Easy and fast: 15 minutes reportable results for large sample screening.
- Instrument interpretation: When equipped with an immunofluorescence analyzer which can eliminate the artificial error and make the result more accurate.
Product Application
- When a clinically suspected patient of COVID-19 with a negative result of nucleic acid test, IgM and IgG antibodies can be used to determine if infected.
- For clinically confirmed cases, dynamic monitoring antibodies levels of IgM and IgG.
- Combined detection of IgM and IgG to improve the detection rate, evaluate the clinical results, and can be a marker for rehabilitative.
Specifications
30 tests/kit





Reviews
There are no reviews yet.